Dr. Shimokawa (CEO, SAGL, LLC) presented our research at the 45th Annual Meeting of the Japanese Society for Basic Gerontology and the Joint Symposium of the Faculty of Human Sciences, Waseda University.
November 24, 2024, Waseda University International Conference Center, Tokyo
Title: Research and development of calorie restriction mimetics: From supporter to player
1. Experience as a supporter of R&D:
During my tenure at Nagasaki University, I worked to form the foundation for drug discovery in academia. In other words, I played a role in supporting faculty and researchers at Nagasaki University. In the 2010s, the Graduate School of Biomedical Sciences organized an education and research group for academic drug discovery. In 2012, Nagasaki University established the Center for Medical Innovation as an organization to practice and support drug discovery1. The University Hospital has decided to become a core clinical research hospital so that investigator-initiated clinical trials can be conducted at high levels. We discussed that the Center for Medical Innovation needed to be unique and different from pharmaceutical companies. Then, we aimed to have the ability to research and develop nanobodies as next-generation antibody drugs and to build a unique library of marine microbial extracts to obtain substance patents when screening hit compounds as the basis for drug discovery. Through the efforts of faculty and staff, the library of marine microbial extracts owned by the Center has been supported by the AMED-BINDS program. Nanobody fabrication facilities have also been established. The University Hospital was approved by the Minister of Health, Labor, and Welfare as a Core Clinical Research Hospital in April 20232.
Several promising R&D projects have been underway at Nagasaki University during this period. The project we at SAGL are interested in is SCM (Stem Cell Memory) Biomedica, Inc., a university-launched venture established by Professor Yoshimasa Tanaka, Director of the Center for Medical Innovation3. Prof. Yoshimasa Tanaka was involved in basic research on PD-1-PD-L1 immune checkpoint under Prof. Tasuku Honjo (Professor Emeritus, Kyoto University, Nobel Prize in Physiology or Medicine 2018) and Prof. Nagahiro Minato (President, Kyoto University). He further developed this research at Nagasaki University and devised a “novel PD-1 immune checkpoint inhibitor combination therapy”. This novel combination therapy is characterized by higher efficiency of cancer treatment and fewer side effects. The company aims to conduct clinical trials in a few years. It is expected to play a significant role in cancer treatment and improving cancer patients' survival rates.
2. Activities as a player for R&D:
The effects of calorie restriction on delaying aging and extending life span have been examined using various animal models. Although it is speculated that it can be applied to humans, it is difficult for humans to voluntarily reduce their food intake for a long time during their social life. Therefore, searching for strategies and substances (calorie restriction mimetics, CRM) that produce the effects of calorie restriction without reducing food intake is one of the most important research topics in aging research. After retiring from Nagasaki University, Shimokawa established a joint venture, SAGL, on January 18, 2023, to research and develop CRMs. Takuya Chiba (currently a professor at Waseda University), Tomoshi Tsuchiya (presently a professor at the University of Toyama), and Shimokawa developed an original system for screening CRMs during his tenure at Nagasaki University4. The idea for this screening system originated from two lifespan studies5, 6. One reported that Ames mice deficient in growth hormone (GH) had an increased life span compared to wild-type mice under the ad libitum-feeding conditions5. The other showed that Ames mice outlived more when CR was performed on them6. Since CR was known to suppress GH, GH suppression was considered an essential component of CR's aging-delaying, lifespan-extending mechanism; the CR study in Ames mice suggested that mechanisms other than GH suppression exist for lifespan extension in CR.
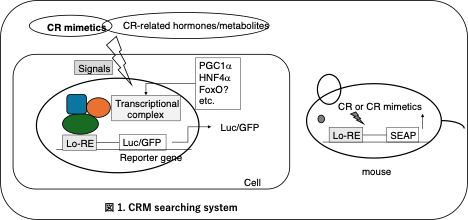
To elucidate this mechanism, Satoshi Tsuchiya analyzed gene expression in the liver of Ames-CR mice in Professor Spindler's laboratory (University of California, Riverside, USA). As a result, he identified a group of genes individually regulated by GH suppression and CR and a group whose expression is additively upregulated7. Tsuchiya predicted that the promoter regions of the additively up-regulated genes share common gene sequences to which transcription factors are bound by GH repression or CR. After returning to Nagasaki University, Tsuchiya collaborated with Chiba and colleagues to show the presence of common sequences to which proteins containing transcription factors bind. Chiba fused a reporter gene downstream of these sequences and constructed an assay system to express the reporter gene when intracellular signals are altered by GH repression or CR and the transcription factor complex binds to these common sequences3. We now call these common sequences the longevity-responsive elements (Lo-RE) and have cells and mice transfected with the Lo-RE-reporter gene as a CRM screening system (Figure 1). A project to discover novel CRMs is underway, which will involve screening the library of marine microbial extracts at the Center for Medical Innovation, Nagasaki University.
3. CRM from the perspective of the Interventions Testing Program (ITP) of the National Institute on Aging (NIA)
In 2002, the NIA began a project to identify substances that extend mice's lifespan and healthy lifespan (ITP) 8). This lifespan test is carried out at three facilities: the University of Michigan, the University of Texas at San Antonio, and the Jackson Laboratory, using the UM-HET3 mouse strain, which has a genetically diverse background. At each facility, 50 male and 50 female mice will be set up as a lifespan group. By integrating the data from the three facilities, the effects of the facility environment and the genetic background of the mice on the lifespan test can be minimized, and sex differences can be identified. Proposals for substances and administration methods, etc., submitted from external research groups or individuals will be reviewed by experts, and about four proposals will be adopted each year. To date, more than 50 substances have been tested 8).
This test is conducted under ad libitum-feeding conditions. Therefore, we can call them CRM if substances significantly extend the median lifespan (the age at which 50% of the mice have died) and maximum lifespan (the age at which 90% of the mice have died) of both male and female mice. Twelve substances extend the median lifespan, including aspirin and glycine 8). On the other hand, only two substances extend both the median and maximum lifespan of both males and females: rapamycin and acarbose. Three substances extend males' median and maximum lifespan but do not affect females: 17-a-estradiol, 16-a-hydroxyestriol, and canagliflozin.
These results suggest that the inhibition of the mechanistic target of the rapamycin (mTOR) pathway by rapamycin and the control of blood glucose concentration, as shown by the effects of acarbose and canagliflozin, are essential factors in controlling aging and lifespan. Although limited to males, the control mechanism by 17-a-estradiol, which has low estrogen activity, and its metabolite 16-a-hydroxyestriol is also a topic for future research.
In the 2000s, multiple mechanisms for controlling aging were proposed using various animal models. For example, lifespan tests based on these hypotheses, such as resveratrol, which activates sirtuins, curcumin, which reduces oxidative stress, and fisetin, which removes senescent cells, have been unable to prove any significant lifespan extension effects. Since lifespan is not the only biomarker of aging, the importance of these substances and hypotheses for regulating aging should not be underestimated. Even if it affects only one or some selected pathways, it may not extend the lifespan. There are also many mouse strains in which the life-extending effects of CR have not been confirmed 9). In the future, to evaluate substances that control aging, it will be necessary to clarify the biological definition of healthy life expectancy and the various biomarkers of aging.
4. Collaborative research centered on Hayashida Financial Management (HFM) and SAGL
We, HFM and SAGL, are already promoting research on the regulation of aging by conducting joint research or providing research support with eight research groups. Among these, the glucosamine project, jointly applied for ITP by five researchers this year, was selected, and within 2025, a lifespan study will begin in the United States10). Glucosamine has been widely used around the world as a supplement or prescription drug to alleviate the symptoms of joint diseases. Reports also suggest that glucosamine is related to aging and lifespan control in humans and experimental animals10). While we wait for the results of the lifespan study on glucosamine, we would also like to elucidate some mechanisms by which glucosamine regulates aging.
References and websites
1. https://www.numic.nagasaki-u.ac.jp
2. https://www.mh.nagasaki-u.ac.jp/research/about/
3. https://www.nagasaki-u.ac.jp/ja/news/news4252.html
4. Chiba, T., Tsuchiya T, Shimokawa I., et al. Development of a bioassay to screen for chemicals mimicking the anti-aging effects of calorie restriction. Biochemical and Biophysical Research Communications 401, 213-218 (2010).
5. Brown-Borg, H. M., Borg, K. E., Meliska, C. J. & Bartke, A. Dwarf mice and the ageing process. Nature 384, 33 (1996).
6. Bartke, A. et al. Extending the lifespan of long-lived mice. Nature 414, 412 (2001).
7. Tsuchiya, T. Spindler S.R., et al. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol. Genom. 17, 307–315 (2004).
8. https://www.nia.nih.gov/research/dab/interventions-testing-program-itp
9. Liao, C.-Y., Rikke, B. A., Johnson, T. E., Diaz, V. & Nelson, J. F. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92 95 (2010).
